Environmental monitoring program documented program implemented through standard operating procedures that describes in detail the procedures and methods used for monitoring particulates as well as microorganisms in controlled environments air surface personnel gear.
Clean room environmental monitoring sop.
5 6 5 the results are calculated and stated as per particle per ft3 fed std 209e.
This would access the environmental conditions in particular the microbiological and particulate quality of the pharmaceutical clean room.
Standard operating procedure title.
As the fda guideline on aseptic processing gmp 2004 states.
In aseptic processing one of the most important laboratory controls is the environmental monitoring program.
Any deviation from this sop is to be recorded in the environmental monitoring comments book and a dr raised if necessary.
The program includes sampling sites frequency of sampling and.
5 6 4 operate the instrument as per sop.
5 6 6 sampling point for non viable air particle count.
Written sops should also address elements such as 1.
Routine environmental monitoring should provide an information base of sufficient size and detail to make decisions regarding the operational status of the area and to ensure that the appropriate level of control is being maintained.
Frequency of sampling 2.
In this context the environmental monitoring data management course by eca academy 20 21 november in barcelona spain will present the basic methodology of evaluating the data using elementary statistical process control tools as well as the empirical approaches to set microbial control limits for cleanrooms.
Environmental monitoring of clean rooms take three one minute one cfm 28 3 liters samples per location for better statistical reliability test laminar flow work stations and barrier isolators the same way testing should be done every six months or after any repairs or renovations.
Personnel see miclab 095 and miclab 060 3 1.
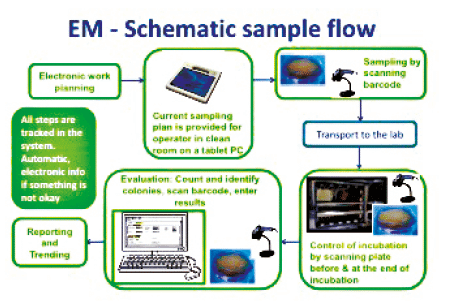
Environmental monitoring em of cleanrooms is the microbiologist s responsibility and it requires making many decisions such as how often to monitor where to monitor what samples to take which culture media to use how long to incubate how to interpret data and which identifications to perform.
When the samples are taken i e during or at the conclusion of operations.
All environmental monitoring locations should be described insops with sufficient detail to allow for reproducible sampling of a given location surveyed.
6 0 abbreviations cfu colony forming unit laf laminar air flow pda potato dextrose agar sop standard operating procedure ipa.
5 6 7 record the result.